What is and how corrosion occurs?
Corrosion
Corrosion is an electrochemical process or chemical degradation, exerted on the surface of metal bodies by the oxygen in moist iar or harsh chemicals.
ISO 9223 Standar classifies the corrosivity of the atmosphere, based on measurements of time and moisture, and the categories of pollution (sulfur dioxide, chlorine, etc.). The Standard is not recommended for use in extreme atmospheres, such as in chemical processing plants.

corrosion on metal surface
Metal corrosion
Corrosion is one of the most important factors determining the degradation of metallic structures. For example, steel corrodes when exposed to salt water. The result is the same in the case of iron in contact with oxygen. These are just two examples. Causes leading to corrosion are numerous, depending on the environment and the substances in the environment.
The process
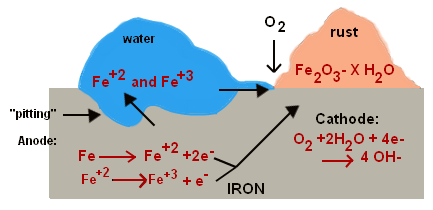
It is complex and varies depending on the material and aggressive elements to which they are exposed. Let's look at a case study related to the corrosion of iron:
- Oxidation of iron: the iron gets oxidized into ferrous ions [Fe (II)] with the loss of two electrons.
Fe →→ Fe+2 + 2 e-
- The ferrous ions again get oxidized into ferric ions [Fe(III)] in the presence of water and oxygen
Fe+2 →→ Fe+3 + e-
- These electrons from the above reactions are used to reduce oxygen.
O2(g) + 2 H2O + 4e- →→ 4 OH-
- The ferric ions combine with oxygen and form ferric oxide [iron (III) oxide]. This ferric oxide gets hydrated with water.
metal structure after corrosion occurs
If you want to find information on corrosion prevention methods, we provide the following contact. Together we will identify the best technical solution to reduce maintenance costs!
Source: http://chemistry.tutorvista.com/physical-chemistry/corrosion.html